Contents
A catalyst is a substance that speeds up a chemical (metabolic) reaction. The catalyst itself is not used up as a result of its actions. Proteins that function as biological catalysts are called enzymes.
They are composed of C, H, O and N. Sulphur (S) may also be present.
As we saw in the nutrition and food webpage the function of proteins is determined by their amino acid composition as well as their shape.
Enzymes control cellular reactions. As you remember, reactions that break down substances and release energy are called catabolic reactions. Examples are respiration and digestion. The other types of reactions are called anabolic reactions. These reactions consume (use) energy. These reactions build larger, more complex, molecules from smaller ones. Photosynthesis and muscle growth from amino acids are examples of anabolic reactions.
ENZYME ACTION
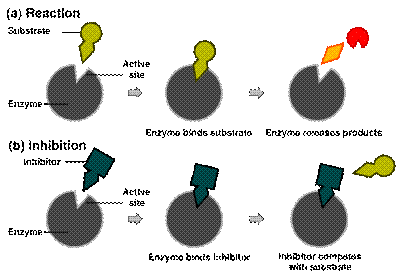
Enzymes are folded in GLOBULAR SHAPES. The enzyme’s shape enables it to receive only one type of molecule; that molecule that will fit into it’s shape. The place where the substance fits into the enzyme is called the active site and the substance that fits into the active site is called the substrate.
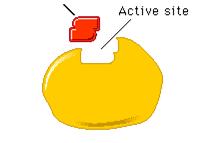
Enzyme action occurs when the enzyme and substrate collide. During the collision the substrate slots into the active site of the enzyme. Collisions happen because of the rapid random movement of molecules.
When the substrate joins with the enzyme the entire structure is called the enzyme-substrate complex. The substrate becomes changed by the enzyme’s action and is then releases as the product. The enzyme is then free to join another substrate.

Enzymes can be either anabolic or catabolic. The same enzyme can be used to form smaller molecules from a larger molecule or to do the opposite.
An example of a catabolic enzyme is amylase. Amylase converts starch into maltose.
An example of an anabolic enzyme is DNA polymerase. This enzyme repairs (rebuilds) DNA.
TYPES OF ENZYME ACTION
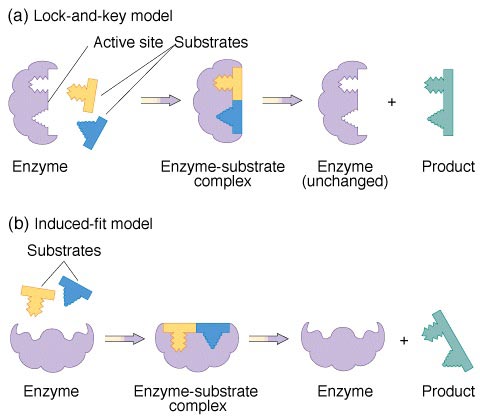
As stated previously, the substrate must fit into the enzyme at the active site. Some substrates fit nicely into the active site. This situation is called the Lock and Key Model.
LOCK AND KEY MODEL
The enzyme is a complex protein molecule, but there is a particular site where the reactant molecule ‘docks in’ by random collision. The enzyme is sometimes referred to as the ‘lock’ and the initial reactant substrate molecule as the ‘key’, hence this is called the ’lock and key’ mechanism. This is also explains why enzymes are very specific (Enzyme Specificity). You need the right molecular key for a particular molecular lock.

Even when different substrate molecules are present, only those that have the specific shape complementary to the active site are able to bind with the enzyme’s active site.
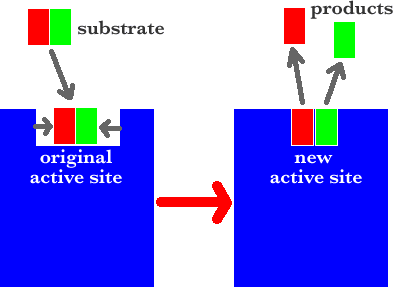
Sometimes the shape of the active site must be slightly changed. This situation is called the Induced Fit Model.
INDUCED FIT MODEL
The enzyme’s active site has a shape closely complementary to the substrate The substrate locks into the active site of the enzyme. The active site alters its shape holding the substrate more tightly and straining it. An enzyme-substrate complex is formed. The substrate undergoes a chemical change and a new substance, product, is formed. The product is released from the active site. The free unaltered active site is ready to receive a fresh substrate.
NAMES OF ENZYMES
Enzymes are named by their substrate. The letters ase are added to the substrates name. Examples are:
lactase – breaks down lactose (milk sugars)
diastase – digests vegetable starch
sucrase – digests complex sugars and starches
maltase – digests disaccharides to monosaccharides (malt sugars)
glucoamylase – breaks down starch to glucose
protease – breaks down proteins found in meats, nuts, eggs, and cheese
lipase – breaks down fats found in most dairy products, nuts, oils, and meat
cellulase – breaks down cellulose, plant fibre; not found in humans
GENERAL EFFECTS OF ENZYMES
Enzymes lower the activation energy. This is the energy input needed to bring about the reaction. Enzymes enable the reaction to occur with less energy than would be needed if the enzyme were not present.
Regulate the thousands of different metabolic reactions in a cell and in the organism.
The activity of a cell is determined by which enzymes are active in the cell at that time.
Cell activity is altered by removing specific enzymes and/or synthesising new enzymes.
INHIBITORS
Enzyme inhibitors are molecules that interact in some way with the enzyme to prevent it from working in the normal manner. Poisons and drugs are examples of enzyme inhibitors. Inhibitors change the shape of the enzyme and make it nonuasable to a substrate. Inhibitors can also act as a substrate and bind to the enzyme. This prevents the enzyme from binding with its intended substrate. When this happens the enzyme is said to be denatured.
FACTORS THAT AFFECT ENZYME ACTIVITY
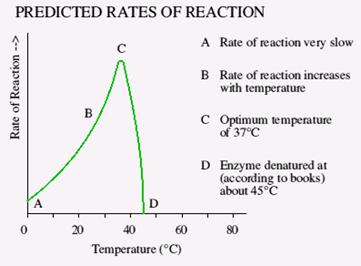
TEMPERATURE
At 0°C enzyme action is low because the movement of molecules is low. This causes the collision frequency between enzyme and substrate to be low. Increasing the temperature speed up the movement of molecules and thus the collision frequency increases therefore enzyme action increases. Human bio enzymes work best at 37 degrees Celsius. As the temperature raises the shape of the enzyme changes and the enzyme becomes denatured. Temperature above 50 degrees Celsius will denature most human enzymes.
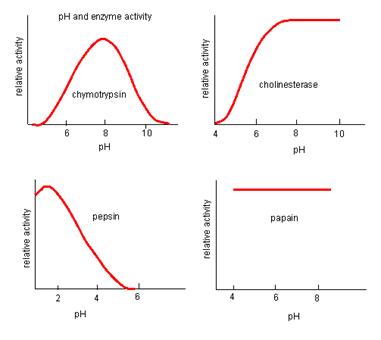
pH
Most enzymes work best at a pH of 6-8. When the pH is outside this range the enzyme will lose its shape and become denatured. The ideal (optimum) pH for most enzymes is 7.
Some enzymes work best at other pH levels. The following graphs demonstrate this:
ENZYME CONCENTRATION
As the concentration of an enzyme increases the rate of reaction also increases, provided that the substrate is in excess.
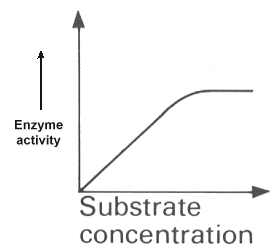
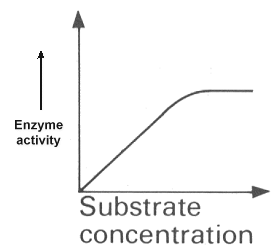
SUBSTRATE CONCENTRATION
At low concentration of substrate an increase in concentration will cause an increase in the rate of reaction. However, once the concentration is such that all the active sites of the enzyme are constantly in use then further increase in substrate concentration will have no effect on the rate of reaction.
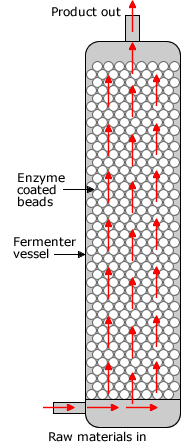
IMMOBILISED ENZYMES
Immobilized enzymes are enzymes which may be attached to each other, to insoluble materials, or enclosed in a membrane or gel. This can provide increased resistance to changes in conditions such as pH or temperature. It also allows enzymes to be held in place throughout the reaction, following which they are easily separated from the products and may be used again.
Immobilised enzymes are used in bioreactors .These procedures are used to produce many products which used to use micro-organisms. See the bacteria webpage for a discussion of bioreactors.
TYPES OF IMMOBILISATION
Adsorption: In this method the enzyme is attached to a support. Supports can be ceramics, glass, or plastics.
Membrane Enclosure: In this method the enzyme is enclosed in a porous membrane.
Gel entrapment: The enzymes are held in a gel. Sodium alginate is a common gel used. The gel allows the substrate to enter and the product to leave.
Chemically bonded to a support or to each other: See textbook page 95.
ADVANTAGES OF IMMOBILISATION
It makes for easier purification of the product as the separation of the enzymes from the products is easily accomplished.
It is easy to recover and recycle the enzymes. This leads to a more economical process.
The enzymes remain functional for much longer as it is a gentler process.
USES OF IMMOBILISED ENZYMES
The following products are derived from immobilised enzyme action:
Fructose derived from glucose: Fructose is sweeter than glucose and is used in soft drinks and other sweet products.
Antibiotics: Enzymes are used to change penicillin into new, wider used, antibiotics.
Sewage Treatment: Instead of bacteria enzymes can be immobilised and used.
Links
Find free Leaving Cert flashcards here.
Just for Fun
Take a break from studying and watch Manchester United! Ireland and UK residents can enjoy four (4) free bets courtesy of Ladbrokes Sports. Learn more about this Ladbrokes promo code at RedeemBonusCode.co.uk.
Find more reviews at PromoCodeLadbrokes.co.uk.